2024 in review: Celebrating biotech innovation and milestones with our clients

Reflecting on 2024, the biotech industry considerably advanced in gene editing, oncology, and AI-driven drug discovery and saw increased M&A activity. We also witnessed a notable shift toward immunology, with innovations from oncology beginning to influence treatments for immunology and autoimmune diseases.
As the year draws to a close, we’re proud to celebrate the milestones of some of our clients, whom we’ve supported at various stages of their business journeys.
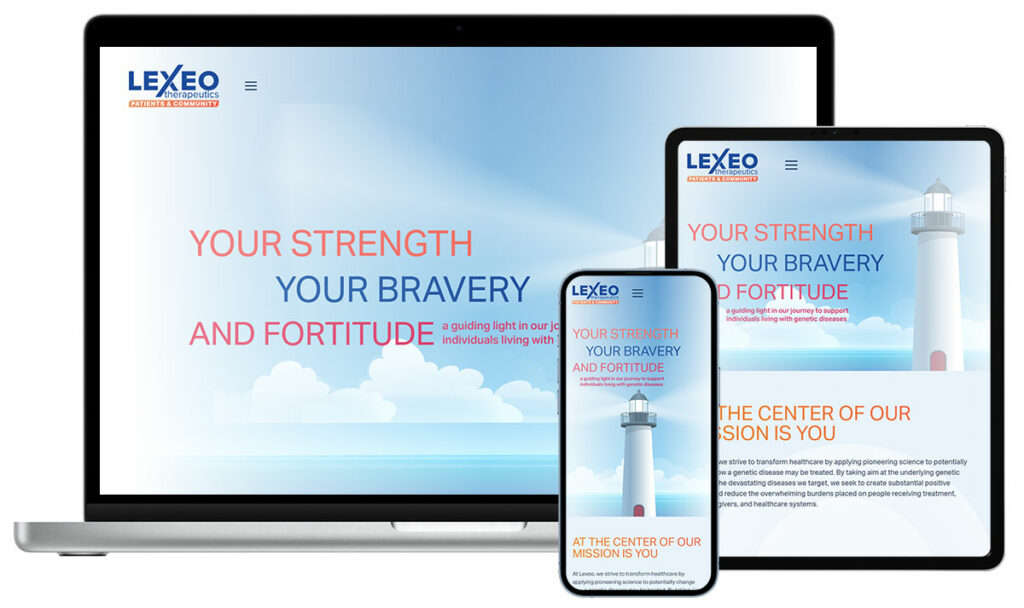
Lexeo TherapeuticsPublic (NASDAQ: LXEO) – Gene therapy for cardiovascular and neurological disorders.
|
 |
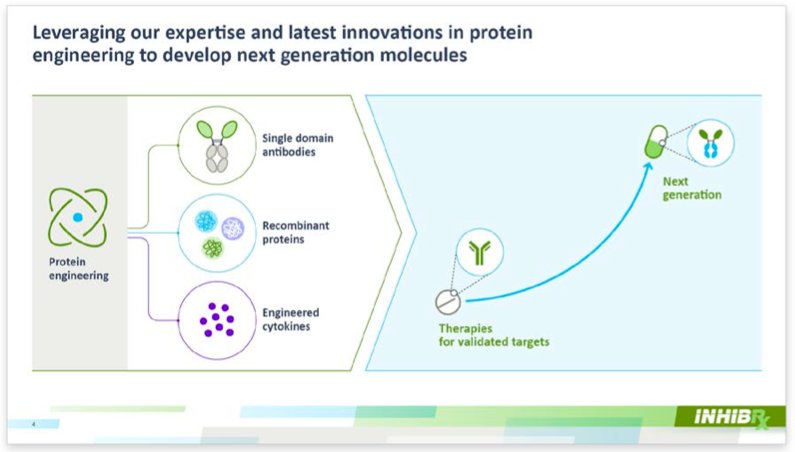 |
Inhibrx TherapeuticsPublic (NASDAQ: INBX) – Next-generation biologics for various diseases. In a landmark move, Inhibrx completed a significant merger by selling their INBRX-101 program to Sanofi. This year, we updated their investor presentation, ensuring it effectively communicates their latest data for key stakeholder meetings. |
DiaMedica TherapeuticsPublic (NASDAQ: DMAC) – Treatments for severe ischemic diseases. Diamedica progressed their lead product candidate, DM199, for acute ischemic stroke to phase 2/3 and dosed the first patient in the phase 2 trial for preeclampsia. We created clear mechanism-of-action (MOA) illustrations and designed a white paper to explain their science. View our work. |
 |
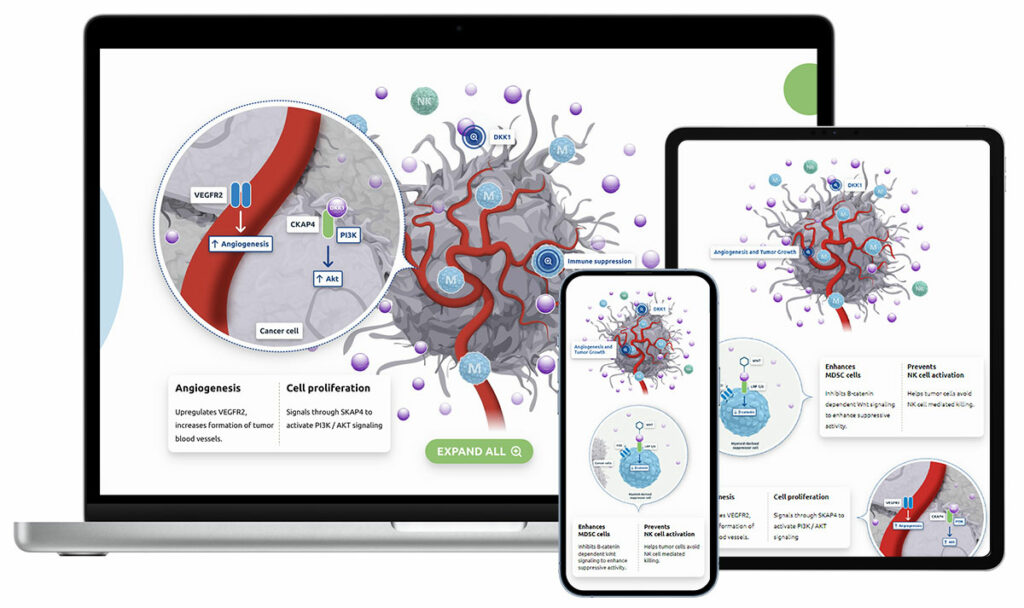 |
Leap TherapeuticsPublic (NASDAQ: LPTX) – Gastrointestinal (GI) cancer immunotherapy. Leap completed enrollment for Part B of the Phase 2 DeFianCe study of their lead product candidate, DKN-01, for the treatment of colorectal cancer patients. We maintained their website and designed MOA illustrations to simplify their science into easy-to-understand visual steps. Visit their website. |
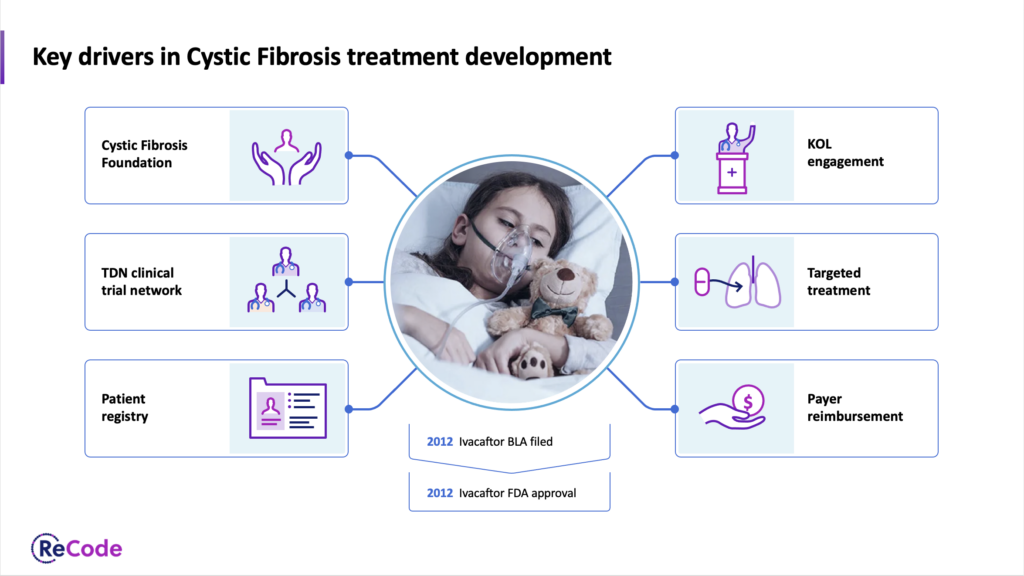
ReCode TherapeuticsPrivate – Genetic medicines for rare diseases using LNP delivery mechanism. ReCode dosed the first patient in their cystic fibrosis clinical trial and secured additional funding from the Cystic Fibrosis Foundation. They also received FDA Orphan Drug Designation for RCT1100, for the treatment of primary ciliary dyskinesia. We help ReCode communicate complex ideas with simple visual slides. |
 |
 |
Tallac TherapeuticsPrivate – Immune-oncology Tallac continued to advance their pipeline of antibody-drug conjugates (ADC) candidates, including novel Toll-like Receptor Agonist Antibody Conjugates (TRAAC). Tallac was listed as one of a few companies to watch that are revolutionizing the ADC industry. A longtime client since inception, we support them with brand creation, LinkedIn management and website maintenance. |
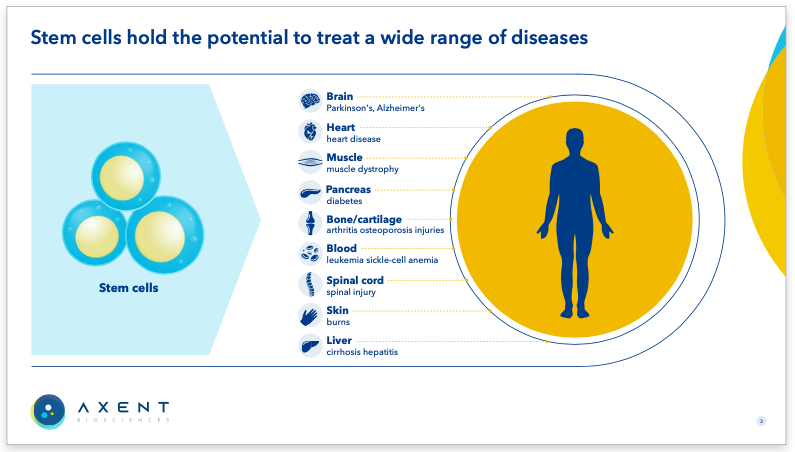
Axent BiosciencesPrivate – Stem cell therapies for unmet medical needs Axent received a $3.99 million grant from the California Institute for Regenerative Medicine (CIRM) to develop a high-quality, accessible cell therapy for Parkinson’s Disease. We designed their brand and created an investor deck, ensuring it effectively communicates their research. |
 |
|
|
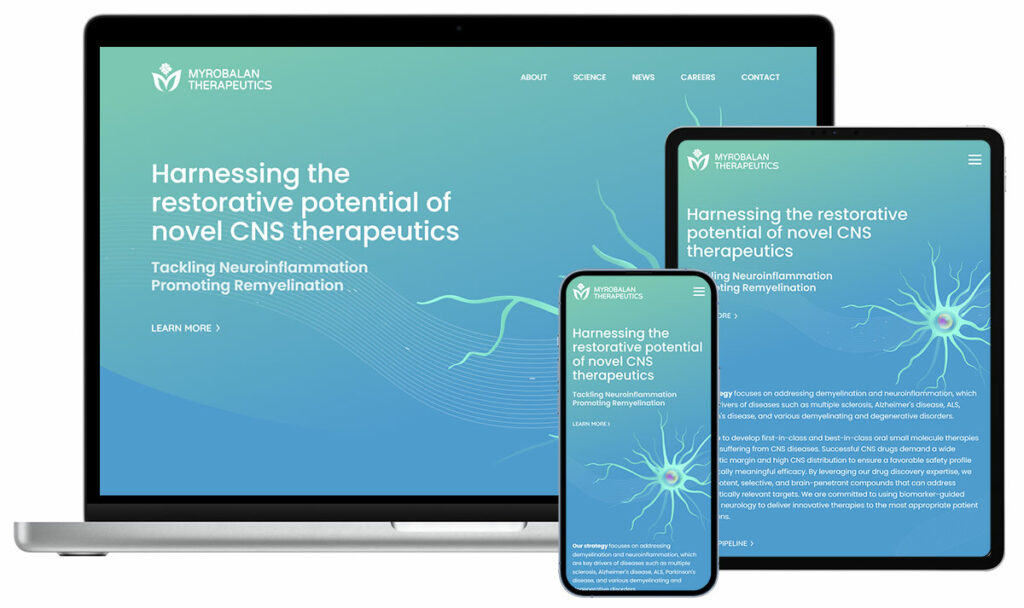
Myrobalan TherapeuticsPrivate – Central nervous system (CNS) disorders, neuro-inflammation and remyelination Myrobalan secured $24 million in Series A financing and a grant from the ALS Association to advance their CSF1R inhibitor. We developed their website, along with detailed MOA illustrations, to showcase their innovative science. |
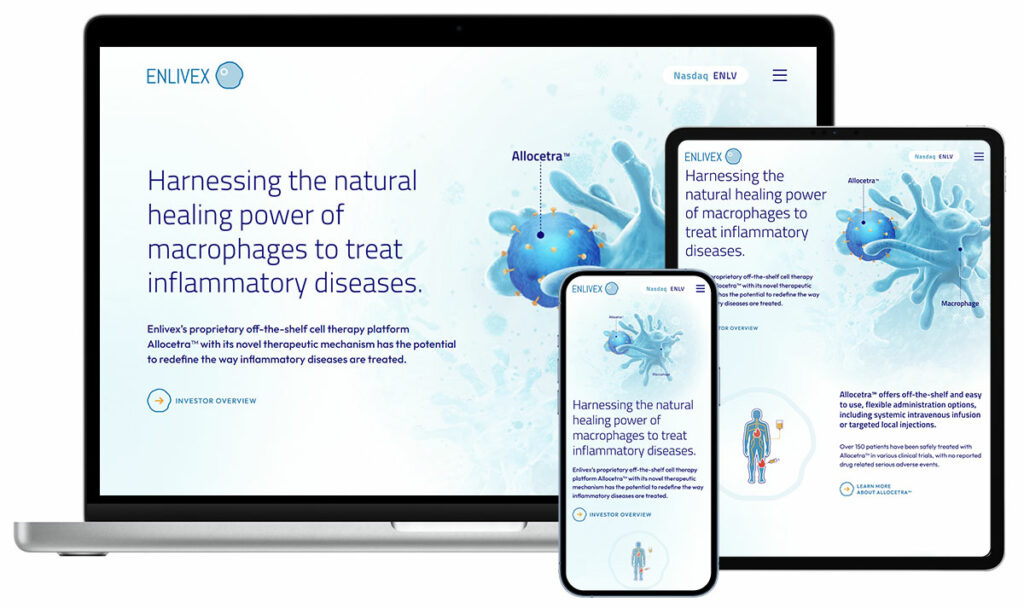
Enlivex TherapeuticsPublic (NASDAQ: ENLV) – Allogeneic cell therapy for inflammatory diseases Enlivex advanced their lead cell therapy candidate, Allocetra™, in osteoarthritis and expanded their pipeline to explore new indications. We developed a story that helped them communicate with investors, created an investor presentation and MOA illustrations and developed a website. |
 |
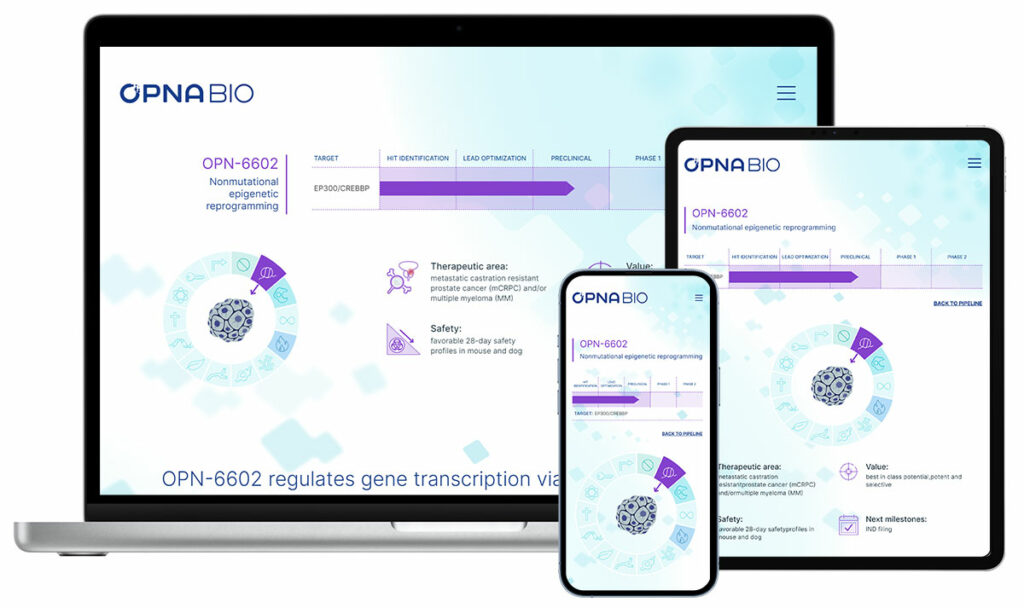 |
Opna BioPrivate – Oncology Opna advanced their programs with the initiation of a Phase 1 trial for OPN-6602 and announced interim data from the ongoing Phase 1 study of OPN-2853. We updated their investor presentation, created scientific posters, and continued to maintain their website with the latest updates. |
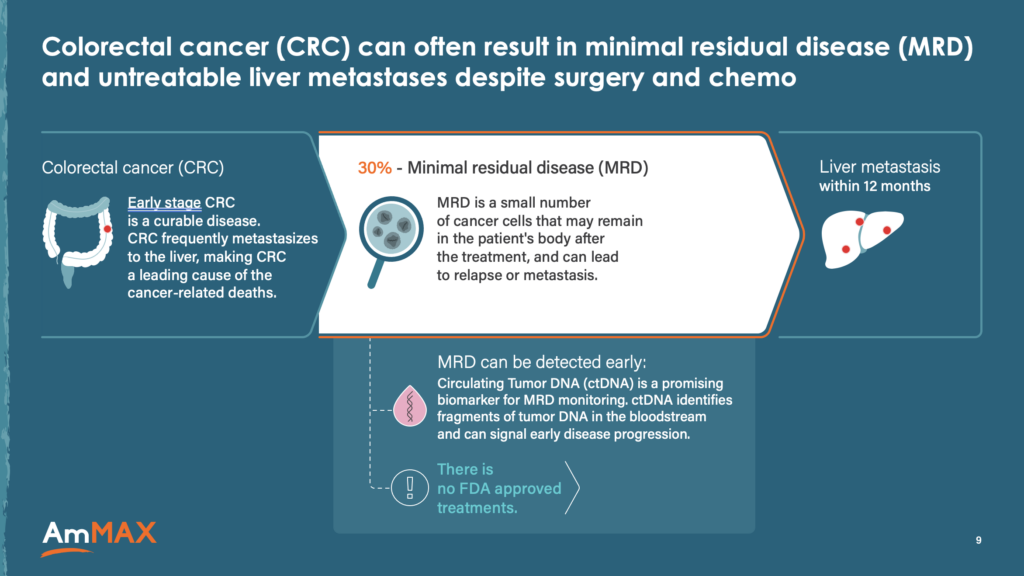
AmMax BioPrivate – Oncology AmMax is collaborating with the University of Texas MD Anderson Cancer Center to advance their lead candidate, AMB-066, for the treatment of colorectal cancer patients with minimal residual disease. We built their investor deck and the story, designed and developed their website, and created simple MOA illustrations to effectively communicate their scientific approach. |
 |
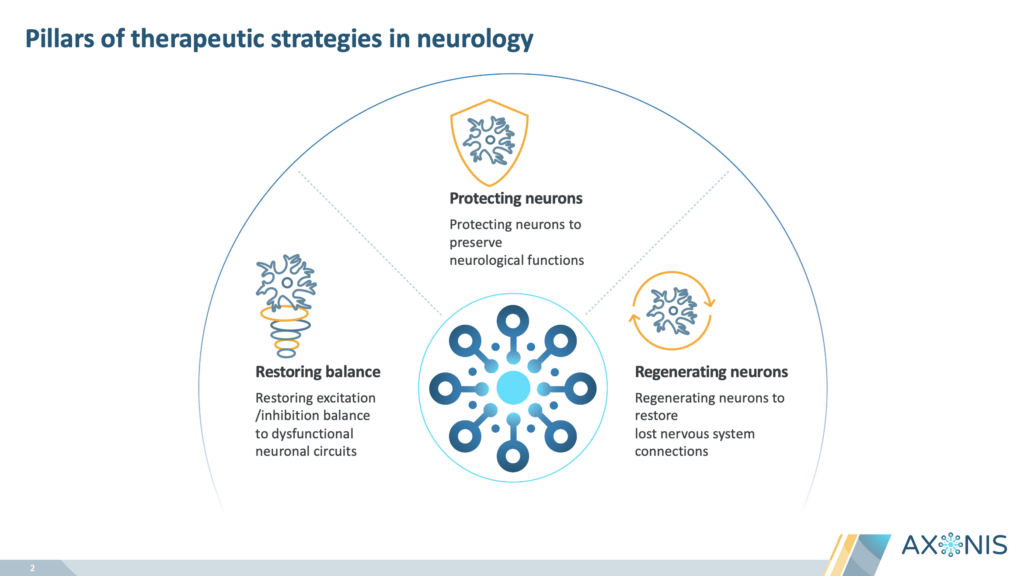 |
Axonis TherapeuticsPrivate – Neurological disorders Axonis closed a $115 million Series A funding round, which will be used to advance their lead development candidate, AXN-027, through clinical proof-of-concept in patients. We helped them tell their story, created an investor deck and developed detailed MOA illustrations to showcase their scientific approach. |
We take great pleasure in witnessing our client’s growth and success as they progress in their drug development journey. As we approach 2025, we wish all our clients the greatest achievements in each stage of their ventures.
Are you preparing for a significant milestone next year that deserves investor attention?
Contact us to schedule your project for next year, whether it’s updating your story and investor presentation, showcasing your scientific approach via illustrative MOA slides or developing a stand-alone pitch on your website.
About Theoria Creative
Theoria Creative is a marketing firm, enabling life sciences companies to clearly communicate complex science to investors, partners, and peers. We develop communication strategy, messaging, positioning, and prepare a set of illustrated materials that help companies clearly and succinctly articulate their value at high stakes meetings.
View examples of our client work here.